What Is Group 7 on the Periodic Table Called
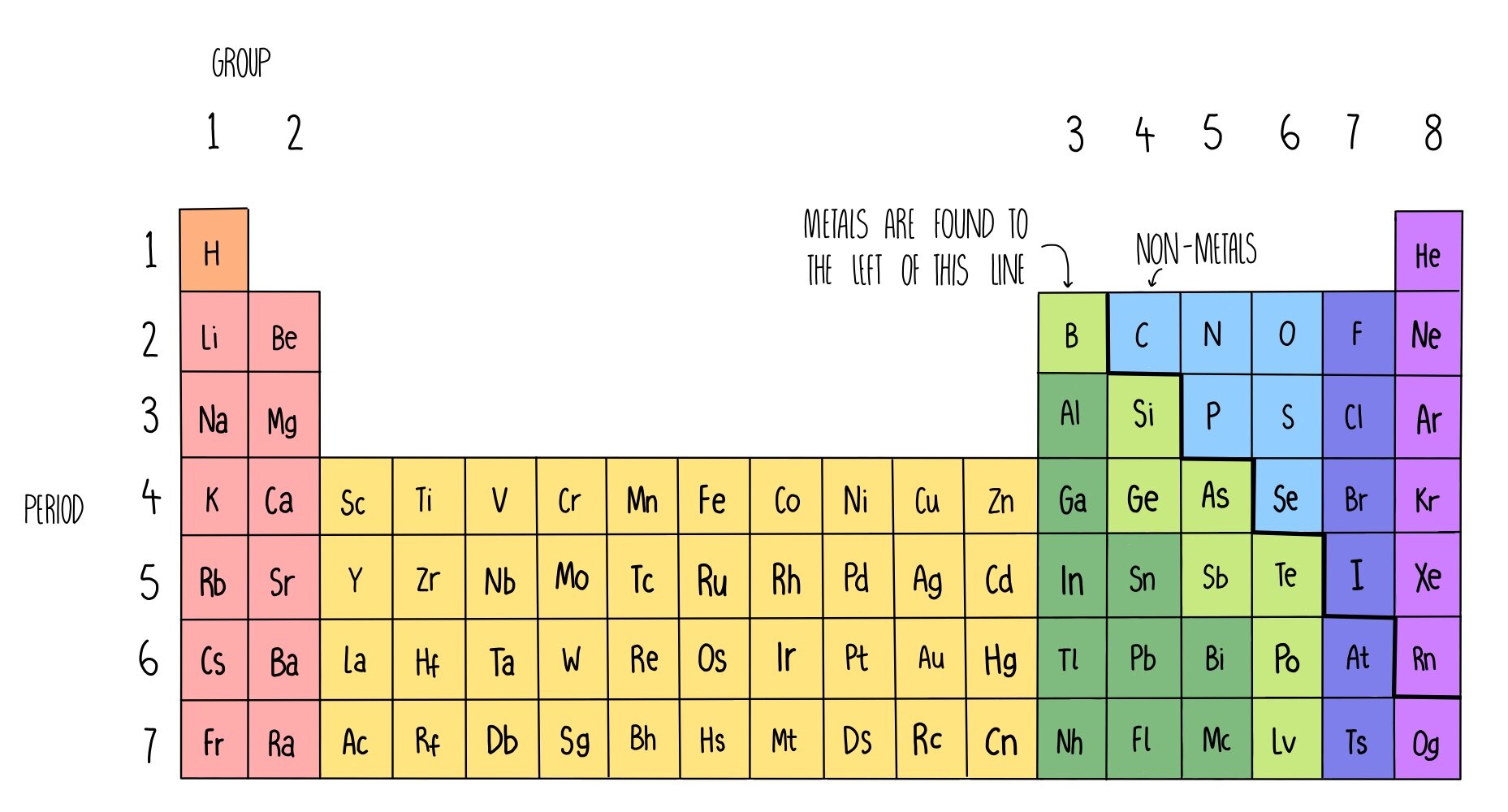
The table has seven rows and 18 columns. Groups 3â12 also known as the âśbâť group elements are called transition.

What Are The Group 7 Elements Called
These elements are found in the crust of the earth and are soft and silvery metals.
. Everything else to the upper right of the staircase plus hydrogen H stranded way back in Group 1 is a nonmetal. All the members of a family of elements have the same number of valence electrons and similar chemical properties. What is group 0 called.
Group 6 numbered by IUPAC style is a group of elements in the periodic table. Metals metalloids non-metals noble gases Valence electrons are. The Group 7 elements are called the halogens.
What are the highly reactive elements in group 17 of the periodic table called. 1 and then classify the element according to its location. The Group 7 elements are called the halogens.
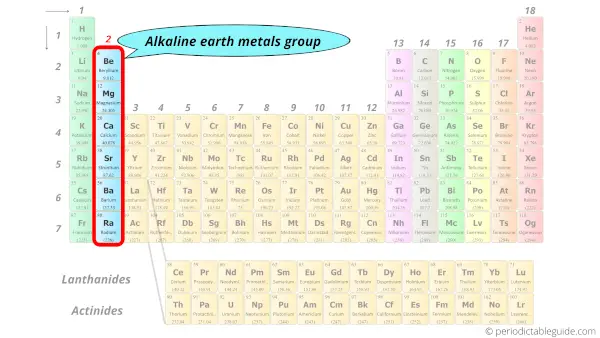
Also what is the name for Group 8 on the periodic table. In a modern periodic table there are seven periods. Some elements in this group are Beryllium Calcium and Magnesium.
They are highly reactive nonmetals with seven valence electrons. Lithium Sodium What is group 7 called. Elements in group 6A are called the chalcogens.
A What is the most common oxidation state of the chalcogens compared to the halogens. Group 7 elements form salts when they react with metals. The two elements combine to form a salt.
That group is now called group 16. What are groups 1 2 and 3 examples of on the periodic table. What are the vertical columns on the periodic table called.
Substance that contains only ONE type of atom. They are placed in the vertical column second from the right in the periodic table. Its members are chromium Cr molybdenum Mo tungsten W and seaborgium Sg.
The Elements In Group 7 Of Periodic Table Are Called Halogens 8x4ezv37xg43 The Periodic Table Chapter 7 Arranging Elements Group 17. The atomic number of selenium is 34 which places it in period 4 and group 16. There are total 18 different groups in Periodic table.
1 selenium lies above and to the right of the diagonal line marking the boundary between metals and nonmetals so. Electrons in the outermost shell all the electrons electrons in the innermost shell The elements in a group have similar properties because they have the same number of protons neutrons valence electrons atoms. Find selenium in the periodic table shown in Figure 27.
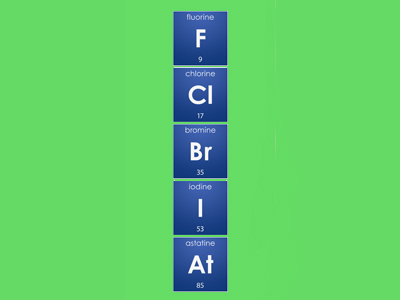
SOLVEDElements in group 7A in the periodic table are called the halogens. The term halogen means salt former which is why Group 7 elements are called halogens. Group 7 elements form salts when they react with metals.
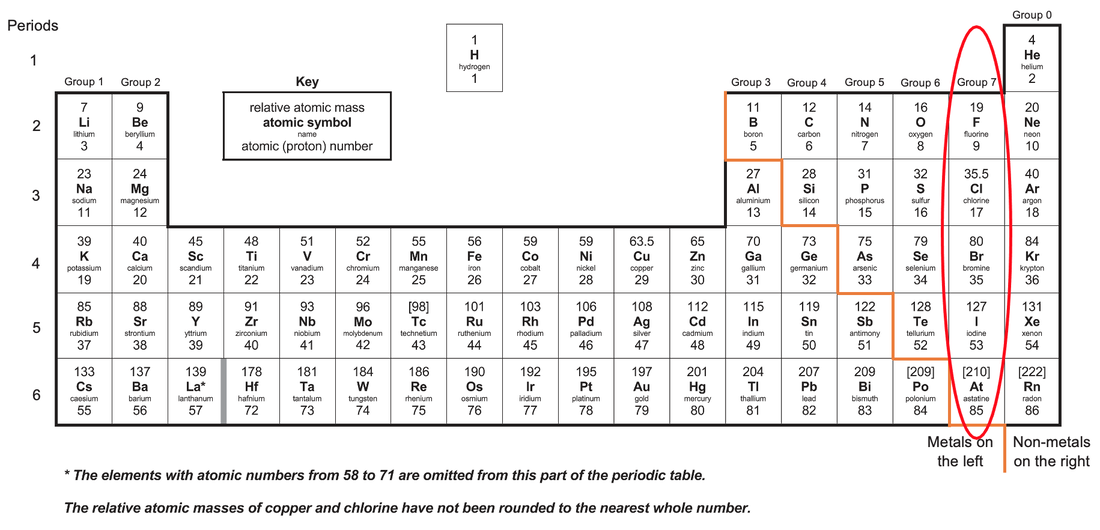
What is group 7 on the periodic table called quest Nonmetals. What is an element. Elements in group 17 are called halogens see Figure below.
A period is any horizontal row of the periodic table and the elements in a period have consecutive atomic numbers. Noble gasses group 8 are the least reactive elements in the periodic table because they have a full valence shellThe right-most column Group 8 or. Chlorine bromine and iodine are the three common Group 7.
Alkaline earth metals group. What is a compound. Group 6 numbered by IUPAC style is a group of elements in the periodic table.
These are all transition metals and chromium molybdenum and tungsten are refractory metals. They can conduct heat and electricity and can be made into sheets. The Elements In Group 7 Of Periodic Table Are Called irp Groups Of The Periodic Table Khan Academy Group 7 Elements The Halogens The Periodic Table The Difference Between An Element Group And Period The Deadly Life Giving And Transient Elements That Make Up Group irp How Are Elements Grouped In The Periodic Table Live Science.
The second group of elements in the Periodic table is Alkaline Earth metals. What is group 1 called. A group is any vertical column in the periodic table and there are 18 such groups.
Chlorine bromine and iodine are the three common Group 7 elements. These include carbon C nitrogen N phosphorus P oxygen O sulfur S and selenium Se. They are placed in the vertical column second from the right in the periodic table.
Alkali metals group hydrogen not included Group 2. Its members are chromium Cr molybdenum Mo tungsten W and seaborgium Sg. The halogens react violently with alkali metals which have one valence electron.
The halogens are so reactive that they cannot exist free in nature. The horizontal rows on. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior.
Transition and Inner transition metals group. These are all transition metals and chromium molybdenum and tungsten are refractory metals.
Pass My Exams Easy Exam Revision Notes For Gsce Chemistry
Periodic Table Groups Explained With 1 18 Group Names
Periodic Table Groups Explained With 1 18 Group Names
The Periodic Table Gcse The Science Hive
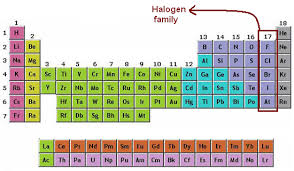
Halogens Fluorine Chlorine Bromine Iodine Astatine

How To Read The Periodic Table Groups Periods Chemtalk

Gcse Periodic Table Revise The Elements In Group Seven

How The Periodic Table Groups The Elements Live Science
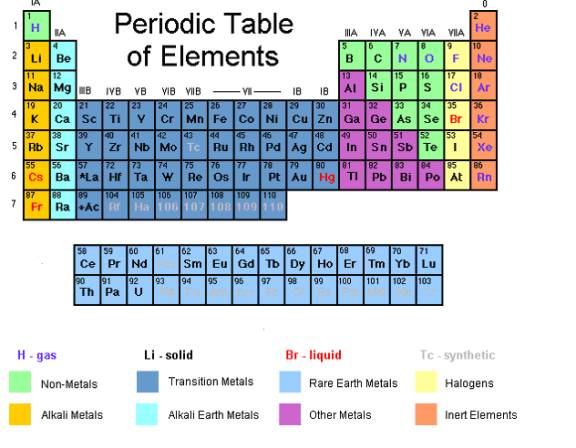
Periodic Table Of Elements With Worked Solutions Videos
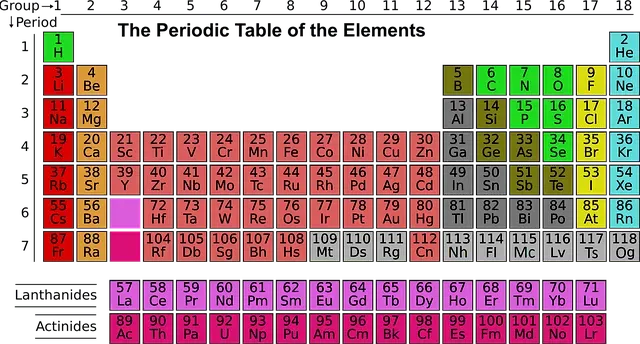
Periodic Table Group Names Explained For Parents
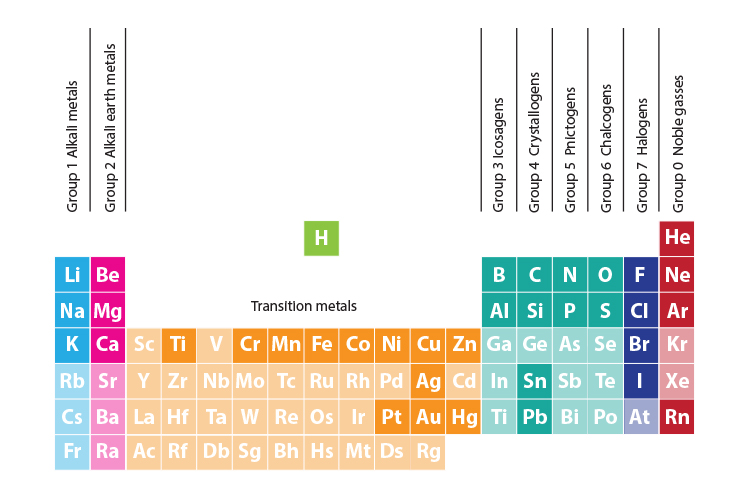
All Elements Of The Periodic Table Are Arranged Into 9 Group

The Periodic Table Chem 1305 General Chemistry I Lecture

What Is Group And Period In Periodic Table
C1 N Group 7 Elements Aqa Combined Science Trilogy Elevise

Halogen Elements Examples Properties Uses Facts Britannica
What Do Elements In Group 2 Of The Periodic Table Have In Common Quora


Comments
Post a Comment